Shanghai artificial heart valve startup NewMed raises over $100 million
Heart disease is an enormous health problem for China, and represents a huge market for medical companies like NewMed and its competitor Hanyu Technology.
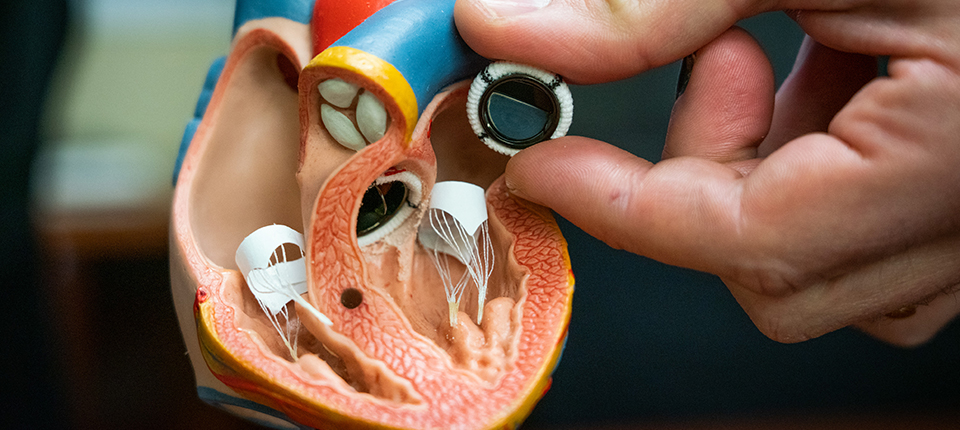
NewMed, a Shanghai-based startup that develops and makes interventional artificial heart valve systems, announced (in Chinese) on Wednesday a Series C funding round of over $100 million.
The funding was led by Singapore state investment company Temasek, followed by Yunfeng Capital, an investment company founded by Jack Ma (马云 Mǎ Yún), OrbiMed, a bio-investment company founded in New York, and five other firms, according to 36Kr (in Chinese).
NewMed, founded in 2015, develops treatment solutions for two of the four heart valves –– the mitral valve and aortic valve.
- The company makes artificial heart valves for transcatheter procedures — a therapy that involves minimally invasive techniques to insert artificial valves to replace existing abnormal or sickened valves when treating heart diseases.
- NewMed now has three artificial heart valve replacement products under clinical trial, one of which has been selected to enter the Chinese drug regulator’s fast-track registration process for “innovative medical devices” and is expected to become the first approved domestically made mitral valve replacement product in China.
China ranks highest in the world in the number and scale of patients undergoing cardiac interventional therapy (in Chinese), and demand for treatments is expected to climb as its population ages. However, China currently relies on imported medical devices used in transcatheter procedures for heart valve diseases.
- Multiple companies and medical experts are trying to develop domestically made artificial heart valves. A team from Zhongshan Hospital of Fudan University, in partnership with Shanghai-based biotech startup Hanyu Technology, completed the enrollment of the last patient in the clinical trial of its transapical mitral valve clamp product — ValveClamp — earlier this month.
- The National Health Commission of China announced (in Chinese) in 2019 that China made progress in the diagnosis and treatment of cardiovascular diseases, saying that artificial hearts independently developed in China would soon be clinically applied.
NewMed, Hanyu Technology, and other Chinese biotech startups developing artificial heart valve replacements will challenge the market currently dominated by global medical device companies focused on cardiac diseases, including Edwards Lifesciences, Medtronic, and Abbott Laboratories.






